Research & Development:
In its early stages, the institute focused on three key research areas: cancer research, family welfare programs, and malaria research. Several anti-cancerous principles have been isolated from medicinal plants, such as solamarine from Solanum trilobatum and plumbagin from Plumbago zeylanica.
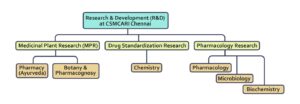
At present, the institute is actively engaged in Ayurvedic research in various disciplines across three broad areas – Medicinal Plant Research, Drug Standardization Research and Pharmacology Research. The focus is on the development and scientific validation of Ayurvedic formulations and single-plant-based medicines.
More than 600 research papers have been published in various national and international journals, 15 book chapters and about 154 scientific papers have been presented in various scientific seminars, conferences and symposia. The work carried out in CSMCARI has been included in the various publications of the Department of AYUSH and ICMR. Research activities confer various departments have been described below. The status of ongoing and completed projects (IMR/EMR) mentioned pertains to last 10 years.
Publication List : Annexure V (Click to open)
4.1. Medicinal Plant Research
Medicinal Plant Research (MPR) at CSMCARI, Chennai, is executed by two departments – Botany/ Pharmacognosy and Pharmacy.
Department of Botany/ Pharmacognosy
Department of Botany/ Pharmacognosy conducts research and provides testing services in various areas related to the identification, authentication, and development of quality standards of medicinal plants and raw drugs used in Ayurveda.
To date, the department has contributed around 50 research publications in national and international journals and successfully completed seven intramural research projects. Four intramural research projects are currently ongoing, focusing on ethnobotany of Kolli and Pachamalai hills, and development of quality standards of drugs used in Ayurveda and traditional medicine. Monographs prepared by the department have been incorporated into the Ayurvedic Pharmacopoeia of India (API) and the Ayurvedic Formulary of India (AFI). The department has developed and published 15 powder monographs in API Part-I, Vol. VIII (2011), 8 Comprehensive Technical Dossiers on single drugs, 117 monographs for Quality Standards of Indian Medicinal Plants (published by ICMR). The department has also carried out studies on several compound formulations including Nimbadi Kwath Curna, Trikatu Curna, Sunthi Guggulu, Gomutra Haritaki, AYUSH-A, AYUSH-D, AYUSH Herbal Tea, and AYUSH M3.
The department also hosts an NABL-accredited laboratory which provides testing services under four parameters: macroscopic identification, sectional studies, powder microscopy, and taxonomic identification of Ayurvedic drugs. Since 2008, the department has been maintaining NABL Accreditation as per ISO/IEC 17025: 2017, adhering to NABL Standards. It is also recognized as a Drug Testing Laboratory (DTL) by the State Government of Tamil Nadu.
Captain Srinivasa Murthy Herbarium (CSMH), the regional herbarium under the department, which houses accessions dating back to 1960, is internationally indexed under Index Herbariorum of New York Botanical Garden, with the acronym “CSMH”. The department provides internships, training, and assistance for dissertations and research projects of post-graduate students. The department is equipped with sophisticated instruments, including High-Performance Liquid Chromatography (HPLC), High-Performance Thin Layer Chromatography (HPTLC), deep freezer (-80°C), a Trinocular compound microscope with CMOS camera and drawing tube attachment, a stereo zoom microscope and a flatbed herbarium scanner (1600 dpi).
Ongoing Projects | Completed Projects |
1. Development of Quality standards for Useful Parts of Selected Medicinal Plants of Ayurveda (IPS0033)2. Development and Digitalization of Authentic Ayurvedic Raw Drugs and Herbarium for the Medicinal Plants Appearing in Ayurvedic Formularies of India in Mandatory Drug Testing Laboratories of CCRAS. (IPS0027)3. Development of Quality Standards of Elsholtzia Species and assessment of Efficacy in Gastric Inflammatory Conditions (CPS0002)4. Medico-Ethnobotanical Surveys in Kolli Malai and Pacha Malai and preparation of Monographs. (IBO0066) | 1. Developing Pharmacognostical Standards for some Ambiguous Ayurvedic Drugs (Sandigdha Dravyas) appearing in the Ayurvedic Formularies, Parts I to III. (IPS0013)2. Pharmacognostical Evaluation of Medicinal Plants cited in Ayurvedic Formulary of India excluding the plants mentioned in Ayurvedic Pharmacopoeia of India- Part –I3. Pharmacognostical Evaluation of Medicinal Plants cited in Ayurvedic Formulary of India excluding the plants mentioned in Ayurvedic Pharmacopoeia of India- Part –II. (IPS0014)4. Photographic Atlas on Macro-microscopic Characteristics of Medicinal Plants Listed in Ayurvedic Pharmacopoeia of India, Part-I, Volume – II. (IPS0019)5. Development of drug Master file and dossier on Fruits of Emblica officinalis (Farm: Phyllanthaceae) for Important Promising Medicinal Plants Based on Leads from Ayurveda & Contemporary Research. (IPS0027)6. Development of Quality Standards of Selected Extra-pharmacopoeial Drugs (Anukta Dravyas) Used in Local Health Traditions Collected from Different Regions of India. (IPS0028)7. Anthology of Pharmacognostical Research Outcomes of CCRAS on Selected Medicinal Plants of Ayurveda. |
Department of Pharmacy/ Ayurveda
The pharmacy department of CSMCARI possesses a pharmacy section and a machinery room. This department is actively engaged in Developing Quality Standards for crude drugs and in developing Standard Operating Procedures for various Ayurvedic formulations. The pharmacy section is equipped with a Pill-making machine, a distillation unit, an End-runner mill, a fermentation vessel, a Tray drier, a Laboratory Blender, a Membrane filter with a vacuum pump, and a Capsule-filling machine. The machinery room is installed with Mini pulveriser (for making curnas), a disintegrator (for making kasayam powders) and a jaw-crusher (for disintegrating very hard plant parts like heart-wood, root and bark).
Ayurvedic drugs are mainly based on plants and plant-based products, besides animal products, metals/minerals and marine origin drugs (Sudha-varga drugs). In the Ayurvedic Drug manufacturing industry, there is a significant increase in information on Ayurvedic drugs and identification of constituents of drugs, parts used, method of preparation and standardisation of products. At the same time, new problems have also emerged in this sector, like non-availability of some of the constituent drugs, especially the root and bark of several plants., increase in demand of Ayurvedic drugs leading to substitution/adulteration, imposition of new laws to protect the environment as well as various plant species and formulations like Curnas (Powders), Bhasmas (metallic preparations), Asavas, Aristas (Alcoholic preparations), according to classical methods. Realising these problems in the supply of raw material, there is a need to facilitate the availability of raw drugs, which are authentic and contain the essential components as required under the Pharmacopoeial Standards. Keeping these facts in view since inception to 2023, the Pharmacy department is working in this research area and has taken up steps to develop Quality standards for plants, animals and mineral origin drugs and develop Standard Operating Procedures for processing to improve the quality of the final product.
The department is also concentrating on ethno-medicinal survey studies (2014-2017). Currently working on developing quality standards for extra-pharmacopeial drugs and Arka formulations and involved in organising medical camps at various places. The department is also acting as the facilitating centre to other departments of the institute, in selecting suitable drugs for research projects. So far, SOPs have been developed for 65 Ayurvedic formulations.
Ongoing Project | Completed Project |
| 1. Ethno-medicinal Survey of Plants Used in Various Diseases by Tribe of Thandarai, Chengalpattu, Kancheepuram District, Tamilnadu2. Developing Quality Standards for selected Extra-pharmacopoeial drugs collected from Irula tribes of Thandarai, Kancheepuram District, Tamilnadu.3. Documentation of Life Profiles of Luminaries of Ayurveda – Vaidyaratna Captain G Srinivasa Murthy4. Documentation of Life Profiles of Luminaries of Ayurveda – Ms Savita Satakopan5. Documentation of Life profiles of Admirable Personalities to Transform Ayurveda (APTA)’- Padmashri Dr.V.Narayanaswamy.6. Documentation of Life Profiles of Luminaries of Ayurveda – Vaidyaratna N Rama Varier7. Documentation of Life profiles of Admirable Personalities to Transform Ayurveda (APTA)’- Aryavaidyan P V Raghava Varier8. Clinical evaluation of Ayurvedic Formulations Shatavari Guda and Triphala kashaya in Swethapradara (leucorrhoea)9. Establishment of Herbal Garden and Nursery centre at RARILSD, Poojapura, Thiruvananthapuram (NMPB-funded EMR project) |
4.2. Drug Standardization Research
Drug Standardisation Unit was established in the year 1979 as a separate project for the Standardization of Siddha drugs. This was consequent to the bifurcation of Central Council for Research in Indian Medicine & Homeopathy into separate Councils in March 1978. Later this unit got merged with the Chemistry department of CSMCARI Chennai.
Department of Chemistry
The department comprises two sections: Analytical and Phytochemical. The Analytical section focuses on standardizing Ayurvedic drugs based on pharmacopoeial parameters. It has completed 446 formulations in Part I and Part II of the Ayurvedic Formulary of India. Standards for individual ingredients used in compound preparations are also being developed. The Phytochemical section investigates the chemistry of medicinal plants through extraction, fractionation, and isolation of known and novel compounds, followed by structural identification and biological screening. Notable bioactive compounds include Solamarine, Gangetin, Vicolides A–D, Physalin D, Arbortristosides A & B, Echitamine chloride, Plumbagin, Cryptanoside-A, Oleanolic acid D-glycoside, and Ruberythric acid. A key achievement is the isolation of an alkaloidal glucoside from Solanum trilobatum, patented and under commercial development for use as a cancer treatment adjuvant.
Since 2008, the department has been maintaining NABL Accreditation as per ISO/IEC 17025: 2017, adheres to NABL Standards. It is also recognized as a Drug Testing Laboratory by the State Government of Tamil Nadu to carry out the drug testing for industries/academic institutes/laboratories etc. In addition to sample testing, the department has been conducting cutting-edge research in the Indian system of medicine, especially, in Ayurvedic sciences. The department has equipped with sophisticated analytical instruments like GC-MS, ICP-OES, HPLC, HPTLC, AAS, Flash chromatography, Flame photometry, UV-Vis spectrophotometer, FT-IR, Rotary evaporator, milli-Q water system, Polarimeter, and other minor equipment. The Department mainly carries out preliminary phytochemical and physicochemical analysis, isolation of chemical markers and their elucidation, development of TLC/HPTLC fingerprint profile, estimation of markers and phytochemicals in single drug as well as compound formulation of herbal drugs, estimation of heavy metals, and identification of adulterants using analytical techniques.
Ongoing Project | Completed Project |
1. Development of quality standards & SOP with marker-based analysis and shelf-life study of four medicinally important classical ayurvedic formulations2. Isolation of Marker Compounds and Development of Marker Compound Library from four Selected Ayurvedic Medicinal Plants (Kancanara, Asthisrnkhala, Puskara & Lajjalu)3. Phytochemical investigation of hydro-alcoholic extractives with different ratios of selected Kvatha formulations4. Pharmaceutical Development and Standards for Formulation AYUSH-64 (Duration: 03 year, 2021-2024)5. Development of Quality Standards, Estimation of Markers, and Shelf-Life Study of Kvatha Curna Formulations (Duration: 03 years) 2023-20266. Evaluation of Seasonal and Geographical Variation on Phytoconstituents/Marker Compounds in Selected Ayurvedic Medicinal Plants (Duration 02 years) 2023-2025.7. Comparative phytochemical evaluation of Ht.Wd. with small branches, leaf, and st.bark of common medicinal plants used in Ayurveda (Duration 02 years) 2024-2026.8. Nutritional profiling, Estimation of marker of Balya Drugs’’9. Development of Quality Standards of Selected Extra-Pharmacopoeial Drugs (AnuktaDravyas) used in Local Health Traditions collected from different regions of India.10. Development of Quality Standards, Estimation of Markers and Accelerated Shelf-Life Studies of Guducyadi, Caturbhadra and Amrtottara Kvatha Curna Formulations.11. Isolation of Markers Compound from Ayurvedic Plants and Development of Marker Compound Library | 1. Estimation of Calcium and Trace minerals in Sudhavarga drugs of Ayurveda.2. Formulation of Drug Master File and Dossier for important promising medicinal plant Emblica officinalis (Phyllanthaceae) based on leads from Ayurveda and contemporary research3. Pharmacognostical Evaluation of Medicinal plants cited in Ayurvedic Formulary of India, Excluding the plants mentioned in API” Part-II” |
4.3. Pharmacological Research
Pharmacological research at CSMCARI, Chennai, is executed by pharmacology, biochemistry and microbiology departments.
Department of Pharmacology
The Pharmacology department is specialized in conducting Pre-clinical toxicity and biological activity evaluation studies of various Ayurvedic formulations. The department has CCSEA (M/o Fisheries Animal Husbandry and Dairying, Government of India) approved Animal House Facility (Reg.No. 591/GO/Re/S/02/CPCSEA) with the total area of 4200 sq. ft. The Department is NABL Accredited as per ISO/IEC 17025:2017 for veterinary testing of hematology samples. Also recognised as a Drug Testing Laboratory by the State Government of Tamil Nadu to carry out the drug testing for industries/academic institutes/laboratories etc and also extending services to scholars and researchers. The lab is equipped with ELISA reader for immunology and hormonal assays, activity cage for excitatory and depressant activity, swimming test apparatus for inducing stress and stress induced ulcerogenic potential studies, metabolic cages for measuring feed and water intake, urine and faecal matter collector, diuretic activity testing, Y maze, plus maze for nootropic activity, histamine chamber for anti-asthmatic activity, veterinary haematology analyser, UV spectrophotometer, Stereo zoom microscope, ECG monitoring, power lab with lab chart software, millar catheter for invasive blood pressure monitoring, CODA monitor for NIBP, Arc HPLC with Empower3 software and nitrogen evaporator for pharmacokinetic studies. In Histopathology facility, the instruments like microtome, hot air oven, incubator are present. The Animal house Facility has a barrier system under CCTV monitoring which contains clean and service corridors equipped with air shower, pass boxes, air handling units, Industrial size Autoclave, RO water facility, UV room for surface sterilization. Isoflurane anaesthesia system is available for small animal procedures and carbon dioxide chamber for animal sacrifice. The Department have MoU with Tamil Nadu Biomedical Waste Management System for biological waste disposal. The department carries out work under Council allotted projects; CCRAS Intramural Research Projects which includes pre-clinical toxicity screening following OECD as well as CCRAS guideline along with other appropriate guidelines such as ICH, EMA etc., and efficacy studies, Collaborative projects with CSIR-IITR Lucknow, ICMR- NIN Hyderabad and many Ph. D. Projects. The department has published several papers in indexed and other reputed journals; received many awards, medals and participated in Oral and Poster presentations in National and International Conferences, Seminars/workshop and scientific meetings as invited and lead speakers. This Institute is affiliated with University of Madras, produced one PhD and currently two scholars are pursuing Ph D in the Department. The staff worked on Ayurvedic formulations such as Parpati, Churna, Vati, Lehya, Bhasma, Guggulu, Extracts, External applications, Single drugs and Eye drops. The department has expertise on animal models like Asthma, Arthritis, Nephropathy, Migraine, Anti-inflammatory, Ocular testing, Surgical model of stroke and Obesity.
Ongoing Projects | Completed Projects |
1. Cardioprotective Effect of Pushkara Guggulu on Experimentally Induced Myocardial Infarction in Rats, instruments purchased, order placed for procurement of animals.2. Development of Madhuca longifolia (Flower) based food recipe (Product development) and its nutritional,toxicity, efficacy and bioavailability studies3. Toxicity profile of Tribhuvana Kirti Rasa in experimental animals.4. Development of Opuntia elatior (fruit) based food recipes (product development) and its nutritional, toxicity, efficacy and bioavailability studies5. Acute and chronic (180 days) oral toxicity study of coded drug AYUSH RP6. Acute and Chronic (180 days) repeated dose oral toxicity study of Nimbatitkam7. Acute and 180-days repeated dose oral toxicity study of Ayush Balrakshak Lehyam | 1. Effect of Haritkadi yoga on middle cerebral artery occlusion ischemic-reperfusion injury and its safety in rats.2. Acute and 90 days repeated dose oral toxicity study of Coded drug AYUSH-PK Avaleha3. Acute and 90 days repeated dose oral toxicity study of Coded drug AYUSH-SDM.4. Development of Drug Master file and Dossier on fruit of Emblica officinalis for promising medicinal plants based on leads for Ayurveda and contemporary research. Pharmacology part.5. Evaluation of Sveta Parpati for its curative and preventive action in Urolithiasis experimental study in rats.6. Preclinical Evaluation (Toxicity and efficacy profile) of an Ayurvedic herbal formulation Vaisvanara Churna used in the treatment of Rheumatoid arthritis.7. Acute and 90 days repeated dose oral toxicity study of Coded drug AYUSH-HR8. In vitro and in vivo nephro protective evaluation of AYUSH K1 extract.9. In-vitro and in-vivo evaluation of anti-migraine activity of AYUSH-M3.10. Safety and anti-obesity activity of Tryusanadi guggulu in experimental animals. |
Department of Biochemistry
Department of Biochemistry was established in 1967. Currently, it is housed on the first floor of the Captain Srinivasa Murthy Central Ayurveda Research Institute, Arumbakkam, Chennai. This department has been accredited with NABL since 2008 with ISO/IEC 17025:2005 and upgraded to ISO/IEC 17025:2017 and strictly follows the NABL norms for quality assurance. This department has good instrumentation facilities. The instruments with periodic maintenance and quality control checks are done regularly to ensure the quality of test results. The department analyses the biochemical aspects of Council allotted safety and efficacy studies and Intra Mural Research projects. This department participates in proficiency testing programs and intra-laboratory comparisons to maintain quality standards. This laboratory is an approved Drug Testing Laboratory for herbal drug analysis by the State Licensing Authority, Indian Systems of Medicine, Government of Tamil Nadu. The deparment supports the postgraduate students for their thesis research work. Several papers have been presented in conferences/seminars and several research articles have been published in well-reputed journals. Besides this, Hands-on training on sophisticated instruments and summer training is regularly provided to undergraduate and postgraduate students from various academic and research institutes. The department was equipped with sophisticated instruments like Clinical chemistry auto analyzer, RT-PCR, Gel Documentation Unit, Western Blot, UV-Visible spectrophotometer, Semi auto analyzer, Tissue homogenizer, Cooling Microcentrifuge, Deep freezer (-20°C), Electrophoresis units, pH meter, Micro centrifuges, Digital Hot top with magnetic stirrer and other accessory instruments.
Ongoing Project | Completed Project |
Anti-cancer activity of Chaulmoogra oil and Shwetha Sarshapa churna in the treatment of epidermal carcinoma of skin in experimental mice. | 1. A biochemical role of AYUSH-PTK, an Ayurvedic formulation against anti-tubercular drug-induced hepatotoxicity in rats (2020-2022)2. Anti-atherosclerotic activity of Hirdayarnava rasa on animal model (2018-2020)3. Biochemical studies on Premna integrifolia Linn. in high-fat diet-induced atherosclerosis model rats (2014-2016) |
Department of Microbiology
The Department of Microbiology at CSMCARI, Chennai, is an NABL-accredited testing laboratory as per ISO/IEC 17025:2017. Routine testing of AYUSH products and DTL samples are conducted in the department in accordance with API guidelines. Along with research activities involving IMR projects. More than 100 Ayush products/samples are being tested in each financial year under IMR projects, and dissertation training/short-duration training is also being provided to the students. The department is equipped with a BSL-2 facility along with various instruments for research activities such as pH meter, Autoclave Bacteriological and BOD Incubators, colony counter, UV-Chamber, Microplate reader, cooling centrifuge, Trinocular Microscope, Cyclomixer, Magnetic stirrer, refrigerator, -40℃ deep freezer and other supporting instruments.
Ongoing Project | Completed Project |
1. Isolation and Identification of Endophytic Microorganisms from the stem barks used in Ayurveda (Panchavalkala) and Evaluation of their Antimicrobial, antioxidant and antibiofilm activities | 1. Antibacterial Activity of selective Ayurveda formulations against Gastrointestinal infection, Respiratory infection and Urinary Tract infection-causing bacteria |